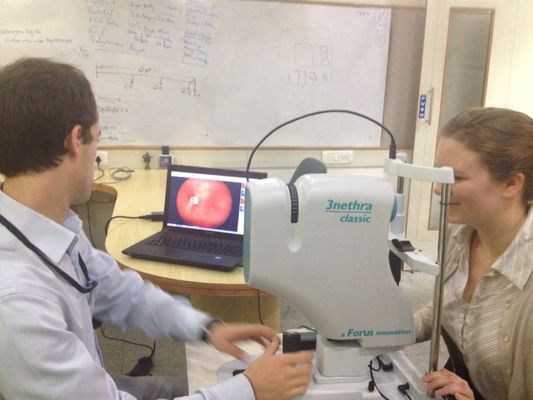
Forus Health, a Bangalore-based Innovations in Healthcare innovator since 2014, develops and sells affordable technology to diagnose eye diseases that can be easily used by minimally trained technicians, making eye care in lower- income settings more accessible. Since 2013, Forus has installed its devices in over 1,100 locations in 25 countries. This year, Forus has set its sights on entering the U.S. market.
Forus’ flagship product, the 3nethra classic imaging device, is an affordable, robust, and portable eye-screening device that allows a health worker to screen a patient in less than 5 minutes for five major eye conditions. To date, over 2 million patients have been screened by the device, which received 510K-exempt clearance from the U.S. Federal Drug Administration (FDA) earlier this year, paving the way for Forus to market the device in the U.S. FDA clearance is important from a manufacturing and quality standpoint globally because it signifies that Forus adheres to the highest international medical device manufacturing standards. The ability to enter the US market is also important from a sales and strategic partnerships perspective.
According to Connor Larkin, who leads U.S. business development for Forus, a large underserved patient base in the U.S. still lacks affordable ophthalmic screening. For example, diabetes-induced blindness (diabetic retinopathy) afflicts over 30% of the 25 million people with diabetes in the U.S. and currently is the leading cause of working age blindness between the ages of 20 and 65. An estimated 50% or more people with diabetes in the U.S. do not receive a medically-required annual retinal screening exam due to lack of accessibility and cost. “In India, we already have around 400 devices linked to a telemedicine-enabled diabetic retinopathy screening network, and plan to replicate a similar system in the U.S. with the goal of ending this type of blindness,” Larkin said.
Since launching in the U.S. in February, Forus has already sold and installed more than 10 devices across multiple states and Puerto Rico. “We plan to pursue multiple market segments, including optometry, opticians, general ophthalmologists, retinal specialists, and teaching hospital institutions – all of which are represented in our current client base – as well as screening at the general physician/primary care level. The goal is to eventually make this type of screening accessible at all income brackets,” Larkin said.
Forus is planning to launch another device, 3nethra Neo, in early 2017. This lightweight device enables clinicians to capture high quality images of neonatal infants’ eyes, and is 1/3 the cost of previous options on the market. Forus Health hopes to be able to launch several more devices in the US by 2018, and to screen 20 million patients within the next three years.